Abstract
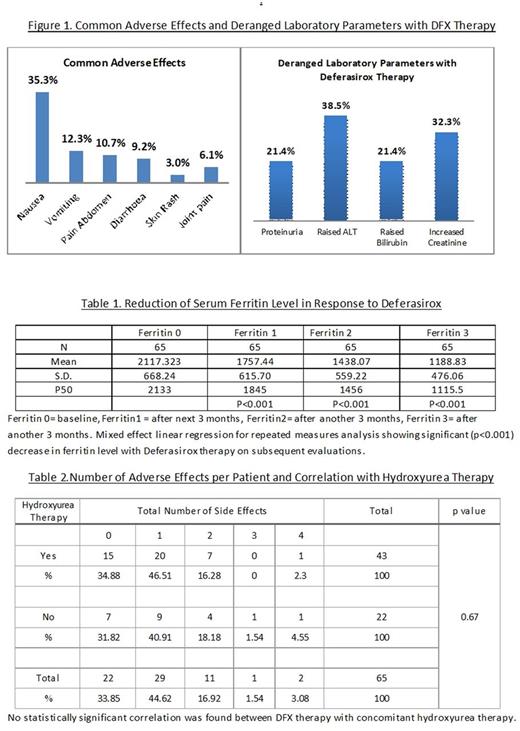
Introduction: In India, more than 10,000 new cases of thalassemia are born every year. In Eastern India, Hb E Beta thalassemia accounts for more than 50 % of the disease burden (Olivieri NF et al, Indian J Med Res 2011;134:522-31). Initially patients were used to get chelation with deferoxamine or deferiprone mostly, but with wide availability of Deferasirox (DFX) it has become the most commonly used chelating agent in Eastern India, however. Limited data are available on chelation from this part of the country. Objective: To assess the response to DFX in chelation-naïve patients with HbE beta thalassemia in terms of changes of serum ferritin level and to document its adverse effects in these patients. Materials and Methods: The study was undertaken from January 2014 to September 2015 at the thalassemia clinic of Institute of Haematology and Transfusion Medicine, Medical College, Kolkata, India. Sixty five patients of HbE beta thalassemia with serum ferritin level >1000 ng/ml and no history of any sort of chelation therapy were enrolled. Evaluation was done to rule out any pre-existing chronic liver disease, renal disease, gastro-intestinal bleed or anaphylaxis. Deferasirox was given at the dose of 40 mg/kg daily to thalassemia major patients and at the dose of 10 mg/kg daily to non-transfusion dependent thalassemia (NTDT) patients. The adverse effects of Deferasirox on HbE beta thalassemia patients were closely monitored. Serial changes in serum ferritin, bilirubin, ALT and creatinine clearance were noted every 3 months. Results: 23% of total patients were NTDT. Mean baseline hemoglobin was 6.72±0.62 gm/dl and mean baseline serum ferritin was 2122±622 ng/ml. Nausea, vomiting, pain abdomen were the most common adverse effects seen (Figure 1). There was no incidence of drug discontinuation or dose reduction. 34% patients had no adverse effects. In rest of the patients, gastrointestinal symptoms were the most commonly associated complaints with DFX therapy. Proteinuria was also found in 21.4 % patients. There was significant reduction of serum ferritin level on repeated measurements. Alterations of bilirubin or ALT were found, but it was statistically not significant. Decrease in creatinine clearance was seen in significant number of patients on DFX. There was no significant association of absence or presence of adverse effects with concomitant hydroxyurea therapy in NTDT patients (Table 2). Discussions: DFX was well tolerated by the patients. Gastrointestinal symptoms, most commonly nausea followed by vomiting and pain abdomen, were the most common adverse effects as seen in other studies like EPIC trial (Ann Hematol 2013 92:211-219). No serious adverse effects like hypoaccusis, GI bleed, anaphylaxis, anasarca as mentioned in other studies (Ali Taher et al. Br J Haematol. 2009;147:752-759) leading to stoppage or dose reduction of DFX were observed. Mixed regression analysis for repeated measures revealed significant decrease in serum ferritin level in comparison to previous ferritin level with DFX therapy (Table No 1), as shown in similar study by Capellini et al (Blood. 2006;107:3455-3462). There was no statistical correlation with absence of any adverse effects when compared with concomitant hydroxyurea therapy, when applicable. Conclusion: Deferasirox was found to be effective in reducing serum ferritin level in Indian HbE beta thalassemia patients without any major adverse drug effects, although more studies with longer duration are needed to achieve robust evidence.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal